|
Site outline: |
|
This level:
|
|
Subpages: |
|
|
|
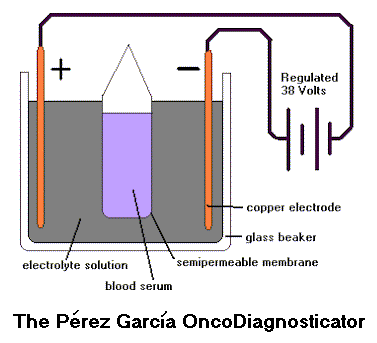
A simple electrochemical method to detect cancer and other diseases? Dr. Donato Perez Garcia y Bellon 2 claimed to have developed just that during the 1950s and 1960s, and used it with his father in their medical practice, until around 1985. A small preliminary study by SGA MD, at McGill University in 1975, found no predictive value. But the method has not, to my knowledge, been tested in any other laboratory. It would be an easy thing to test. And if it turns out to have any merit whatsoever, it could be researched, improved, and repackaged with modern optics and electronics to provide an inexpensive early diagnostic test for cancer and other diseases. The OncoDiagnosticator, as they called it, or the Onco, for short, was extremely simple. Any high school student could make one for a science project.
Take a glass container -- perhaps a 50 ml beaker. Fill it with a neutral (pH = 7) electrolytic solution. Place bare copper wires in the solution at opposite sides of the container. Put a few milliliters of a patient's blood serum in a sleeve, pocket, or envelope made of semipermeable membrane, and hang it in the middle of the solution. Finally, connect the copper wire electrodes to a regulated 32 volt power source and wait for two hours. What could be simpler?
Dr. Perez Garcia y Bellon 2 found that the serum would often change to a violet color, readily visible when the serum was poured into a test tube at the end of the reaction. Based on his experience, he believed that absence of violet would mean absence of cancer, pale violet would mean susceptibility to cancer, darker violet would indicate early-stage still-hidden cancer, and very dark violet would indicate the presence of later-stage cancer discoverable by standard methods. Later work found other colors, again seeming to correlate with the type and severity of disease. Although for him the color was the primary indicator, he also found that the pH of the electrolyte solution in the beaker would change depending on the patient's condition. In healthy people the pH would be below 8.5. And in cancer patients the pH would be higher: 8.5-9.5 or more in males, and 9.5 to 10.5 in females. He also found that the temperature of the electrolyte solution would become higher in cancer patients than in normal people, and would be higher in female cancer patients than in male cancer patients. I assume that higher temperature means higher current flow, and indeed Dr. Perez also measured current (milliamps). Dr. Perez never knew exactly what was going on chemically in the system. As with IPT itself, he just found empirically that it worked. Dr. Perez Garcia y Bellon 2 told me that the color, pH, and temperature would move closer to normal as a patient underwent weeks of IPT treatments. So apparently the method could be used to monitor the progress of a patient's condition. Does the Onco really work? And if so, what is going on inside that beaker? And when will a researcher get interested enough to find out?
Materials details:
Technique details:
The doctors Perez 1 and 2 determined color by comparison to a color chart. But it could also be measured with a colorimeter. Publications: Dr. Perez Garcia y Bellon 2 distributed (but did not publish) two papers relating to the Onco. The first is a basic description. The second paper develops further his theory, based on using the Onco, that chronic degenerative diseases can be correlated with the content of nutrient and toxin elements in the blood. He and his father also discussed the Onco in chapter 12 of their book Cellular Cancer Therapy Through Modification of the Blood Physico-Chemical Constants (Donatian Therapy). |